Uganda has scored a major win in the fight against HIV after the National Drug Authority (NDA) officially approved lenacapavir, a long-acting injectable drug for HIV prevention that is administered just twice a year.
In a statement confirming the decision, the NDA described the approval as a “game changer,” saying the new prevention option could significantly strengthen efforts to reduce new HIV infections, especially among people at high risk, as the country works toward ending AIDS by 2030.
Lenacapavir is manufactured by US-based biopharmaceutical company Gilead Sciences and belongs to a new generation of HIV prevention medicines known as pre-exposure prophylaxis (PrEP). Unlike the commonly used daily PrEP pills, lenacapavir is given as an injection only once every six months, a shift experts say could dramatically improve adherence.
“This is a game-changer for HIV prevention, especially for those at high risk. This is a great step towards ending AIDS by 2030,” the NDA said in a post on X (formerly Twitter).
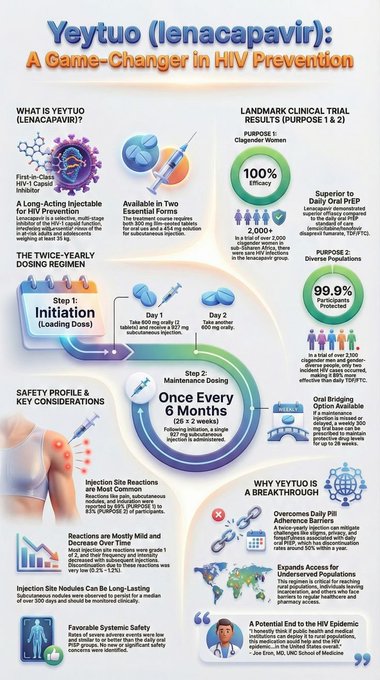
The injectable is designed for adults and adolescents at substantial risk of HIV infection, offering a discreet and convenient alternative to daily medication. Health specialists believe this could help overcome long-standing barriers such as pill fatigue, stigma associated with taking HIV-related drugs, and the challenge of regular clinic visits.
The approval is backed by strong scientific evidence. Clinical trials that informed regulatory decisions showed near-complete protection against HIV infection when lenacapavir injections were taken as scheduled. In June 2025, the US Food and Drug Administration approved the drug for HIV prevention under the brand name Yeztugo, after studies showed a 99.9 percent reduction in infection risk among users.
Uganda’s move places it among a growing number of African countries embracing long-acting HIV prevention technologies. Regulatory submissions and approvals are ongoing across sub-Saharan Africa, including South Africa, Kenya, Zambia, Rwanda, Tanzania, Zimbabwe, Botswana, Malawi, and Namibia, as partners work to expand access through collaborations with generic manufacturers.
Public health experts say the addition of lenacapavir to Uganda’s HIV prevention toolkit could be especially impactful for young people, women, and key populations, who continue to face higher infection risks.
BREAKING: Uganda’s National Drug Authority has just approved Lenacapavir, a twice-yearly dose PrEP manufactured by Gilead, a USA based company! This is a game-changer for HIV prevention, especially for those at high risk. This is great step towards ending AIDS by 2030. pic.twitter.com/tjzQoZucXn
— Uganda National Drug Authority (@UNDAuthority) January 5, 2026